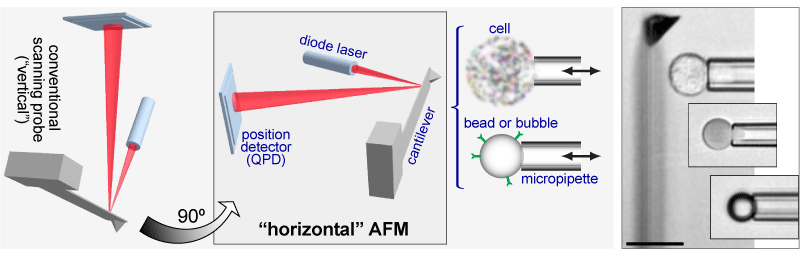
Horizontal AFM
Jump to: Recent Publications
|
Why do we need this kind of AFM? [adopted from the Introduction of Ounkomol et al. 2009] |
|
| Elastic microbeams were used as early as 1980 to inspect piconewton “cellular forces” [Evans et al., Cell Biophys. 1980]. The same concept became the core of the now widely used atomic force microscope (AFM), which originally was designed as a scanning probe to map physical and/or chemical properties of test surfaces into high-resolution images [Binnig et al., Phys. Rev. Lett. 1986]. In addition to its predominant role as an imaging device, a properly calibrated AFM can also apply and report minuscule forces. We currently use our AFM exclusively in the latter capacity. |
| Changes in AFM-cantilever deflection can be measured with exquisite resolution by tracking a detection-laser beam that is reflected off the back side of the cantilever (“optical-lever method”). However, accurate force measurements with relevance to nano- and micro-scale biological function require special care and remain a veritable challenge, in particular when dealing with objects in solution such as live cells or biomolecules. For example, the range of forces that are accessible with a single cantilever is limited. In live-cell studies the cells are often immobilized on the substrate or cantilever surface. It is largely unclear how the biochemical interactions that “glue” a cell to the carrier surface affect the forces measured at its opposite side. Irregular cell geometry and an elevated, unknown cortical tension cause additional uncertainties in the results of such studies. Further, relative movement of an extended substrate to/from the cantilever produces considerable bias forces due to hydrodynamic coupling. |
| Addressing most of these limitations, our force probe is designed for mechanical tests on objects submerged in an aqueous solution with a primary focus on biological samples. It entails two main innovations. First, the core components of the force module—a cantilever, a focusable diode laser, and a photodetector—are mounted in a “horizontal” configuration on the motorized stage of an optical research microscope. This arrangement provides a “side view” of ongoing nanomechanical experiments while retaining the high spatial and temporal resolution of the optical-lever-based cantilever-deflection measurement. Second, this configuration has allowed us to combine the force module with a micropipette-manipulation system. The principal idea of this setup follows the “pre-AFM” footsteps of what appears to have been the first cantilever-based piconewton force probe [Evans et al., Cell Biophys. 1980], upgrading the early design with an optical-lever module, microfabricated cantilevers, and a high degree of automation. |
__________________________________________________________________________________________________________
RECENT PUBLICATIONS USING THIS UNIQUE AFM
| 2010. Ounkomol, C., S. Yamada, and V. Heinrich. Single-cell adhesion tests against functionalized microspheres arrayed on AFM cantilevers confirm heterophilic E- and N-cadherin binding. Biophysical Journal 99:L100-L102. doi:10.1016/j.bpj.2010.11.013 || PDF 369 KB |
 |
2009. Ounkomol, C., H. Xie, P.A. Dayton, and V. Heinrich. Versatile horizontal force probe for mechanical tests on pipette-held cells, particles, and membrane capsules. Biophysical Journal 96:1218-1231. doi:10.1016/j.bpj.2008.10.047 || PDF 1593 KB
| 2008. Heinrich, V., and C. Ounkomol. Biophysics in reverse: Using blood cells to accurately calibrate force-microscopy cantilevers. Applied Physics Letters 92(15):153902. doi:10.1063/1.2909529 || PDF 616 KB |
 |